Commentary: 4-Bromochlorobenzene—A Pillar in Halogenated Aromatic Chemistry
Historical Development
4-Bromochlorobenzene, a simple halogenated benzene, cropped up in chemical catalogs more than a century ago. Early chemists eyed its dual halogenation and wondered about the balance between reactivity and stability, comparing it to its monochlorinated and brominated cousins. New synthetic methods in the twentieth century, especially improved routes to selectively halogenate aromatics, made commercial-scale production possible. The compound started popping up not only in textbooks but also found homes in organic synthesis labs, where researchers chased new materials and pharmaceuticals. The past sixty years, packed with research into aryl halide cross-coupling and polymer chemistry, showed just how valuable such a scaffold could be for constructing complex molecules.
Product Overview
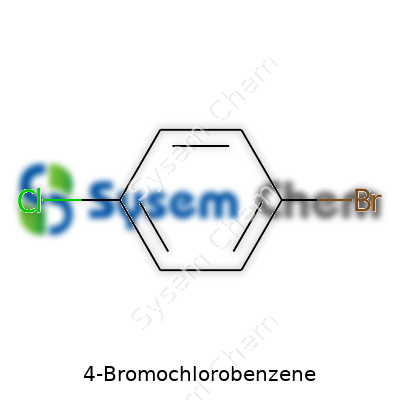
4-Bromochlorobenzene’s structure—an aromatic ring with a bromine at the para position to a chlorine atom—gives it a unique spot among aromatic halides. It slots into roles ranging from building blocks for advanced chemicals to reference standards in laboratories. Chemical suppliers count it among their core halobenzenes, and its relative simplicity makes it a staple in the toolkit of synthetic chemists. At the bench, anyone developing a new catalyst, or mapping out a reaction mechanism involving electrophilic aromatic substitution, will have handled material like this. Bulk users in the pharmaceutical and specialty materials world count on reliable, high-purity product that fits into demanding downstream processes.
Physical & Chemical Properties
This compound takes a crystalline, white-to-off-white solid form under normal conditions. Melting starts close to 54°C, with boiling nudging 242°C. Its modest vapor pressure and low volatility mean it isn’t popping out of bottles like some halogenated solvents. The density, around 1.6 g/cm³, registers typical for this class of molecules. Solubility drops in water to negligible levels, but it finds compatibility with nonpolar organics—benzene, ether, and carbon tetrachloride among them. Chemists note its resistance to acid and base, making it handy in multi-step syntheses. Reactivity centers on the aromatic ring; both halogens behave as leaving groups in nucleophilic aromatic substitution once conditions get strong enough.
Technical Specifications & Labeling
Sourcing for research or manufacturing calls for close attention to purity—most commercial bottles tout 98% or greater, with water and related halobenzenes as typical contaminants. Labels often provide CAS number 106-39-8, along with boiling and melting points, hazard pictograms, and recommended storage: cool, dry, out of sunlight. Shelf life can stretch for years with proper sealing, no metal or heat exposure. Size options range from gram-scale vials for academic labs to kilogram drums for process chemistry. Certificates of analysis go deep—trace metals, moisture content, retention time—because trace impurities may throw off sensitive reactions or toxicology studies.
Preparation Method
Making 4-bromochlorobenzene cleverly exploits both regioselectivity and reactivity. Many producers start with chlorobenzene and use bromine under tightly controlled Lewis acid conditions—iron(III) chloride, for example. Reaction temperature, rate of addition, and mixing all determine whether the para or ortho product dominates. Alternative routes rely on copper-catalyzed halogen exchange, or even direct halogenation of benzene, but these tend to produce more waste and less precision. Modern practitioners focus on yield and green chemistry: solvent choice, recycling, and minimization of byproducts have become routine points of optimization.
Chemical Reactions & Modifications
Chemists approach 4-bromochlorobenzene as a two-handed tool for arylation and cross-coupling. The bromine reacts markedly faster than the chlorine in palladium-catalyzed Suzuki, Stille, or Buchwald-Hartwig reactions, creating a path for stepwise functionalization. In another pot, both substituents can be swapped via nucleophilic aromatic substitution—or left in place as rugged, electron-withdrawing groups. Laboratories have harnessed it to explore aromatic substitution selectivity, especially in the context of biaryl synthesis for drug discovery. Some polymer chemists use 4-bromochlorobenzene as an initiator or monomer, playing off the different reactivity of its two halogens to build up complex chain architectures.
Synonyms & Product Names
Chemical references list a raft of names: para-bromochlorobenzene, 1-bromo-4-chlorobenzene, 4-chlorobromobenzene, and even PCB4 (not to be confused with polychlorinated biphenyls). International trade uses the primary IUPAC name, but regional catalogs may favor the alternative arrangements. Specialty suppliers sometimes market this product under branded names to indicate high-purity or certified grades for regulated applications.
Safety & Operational Standards
Many handle 4-bromochlorobenzene with a respect born of routine, but safety data force a pause. Direct skin or eye contact may cause irritation; inhalation of dust in large quantities can irritate mucous membranes. Glove use and lab coat remain musts, with good ventilation or fume hoods protecting against vapor at elevated temperatures. Waste treatment calls for incineration or a licensed disposal contractor, keeping halogenated residues out of waterways. Safety protocols, shaped by GHS and REACH, require regular review—especially for workplaces with new staff or less experience. Some jurisdictions mandate environmental controls for bulk use, fearing halogenated aromatic pollution.
Application Area
Industries draw on 4-bromochlorobenzene mostly as a starting material for dyes, agrochemicals, and pharmaceuticals. Medicinal chemists reach for it to build diaryl ethers and aryl amines—motifs found in next-generation antihistamines, kinase inhibitors, and more. Certain specialty polymers rely on its bifunctional nature, especially as researchers push halogen-rich polymers for electronics or ion transport. Analytical labs deploy it as a reference or standard in purity testing by GC/MS and HPLC. The demand for bespoke ligands and small-molecule scaffolds ensures steady interest, both in research and process chemistry.
Research & Development
Ongoing R&D homes in on the balance between reactivity and selectivity. Catalysis experts keep hunting for less toxic, recyclable phosphorus ligands or base metals to swap bromine or chlorine cleanly, yet cost-effectively. Environmental teams analyze how these halogenated benzenes move through soil or degrade in sunlight, trying to close loopholes in waste disposal or accidental releases. Computational chemists simulate interactions to predict outcomes of couplings or substitutions, guiding others away from time-wasting dead ends. Some groups chase biotransformation, using engineered microbes to break down similar aromatics—and teasing out pathways relevant for bioremediation or green synthesis.
Toxicity Research
The story around 4-bromochlorobenzene’s toxicity reminds us of the broader scrutiny aimed at halogenated organics. High doses in rodents produce liver and kidney stress, while low, acute exposures show little systemic uptake. Chronic effects and long-term accumulation have not matched the alarm raised by polychlorinated biphenyls, but research stresses caution in occupational settings, especially for personnel with daily handling. In aquatic systems, persistence draws more worry—bioaccumulation remains low, but breakdown pathways spawn metabolites needing further study. Most published reports argue for strict engineering controls and regular health monitoring for repeated users.
Future Prospects
Looking ahead, 4-bromochlorobenzene keeps its grip as a flexible chemical “platform molecule.” Demand for high-value pharmaceuticals, advanced materials, and greener syntheses all pull for reliable, cost-efficient sources. Regulatory pressure on halogenated compounds has sharpened focus on responsible manufacturing and safe use. Advances in catalysis, especially those phasing out precious metals, might tilt scales toward new derivatives or help unlock tougher transformations. Researchers and regulators share a stake in tracking environmental movement and residue profiles, as global chemical safety standards keep rising. Whether in the hands of synthetic pioneers or industrial producers, 4-bromochlorobenzene looks set to power new reactions and keep laboratory imaginations busy for another generation.
Understanding 4-Bromochlorobenzene
4-Bromochlorobenzene doesn’t get attention outside of chemical circles, but its role in industry and research goes deeper than folks realize. With a pretty straightforward structure—a benzene ring with one bromine and one chlorine atom—it slips easily into a bunch of reactions chemists run daily.
Why Industries Reach For It
People working in laboratories see compounds like 4-Bromochlorobenzene as building blocks. You want to make something more complex? You start with simple pieces. This chemical finds frequent use crafting new materials, pharmaceuticals, and agrochemicals. Its special arrangement lets researchers swap the bromine or chlorine for new groups without much fuss, speeding up how quickly projects move from planning to reality.
Every experienced bench chemist I’ve spoken with talks about the value of simple substitution. In medicinal chemistry, this approach shortens the leap from concept to candidate drug, saving months—even years—when you’re racing a disease or a competitor.
Bringing New Molecules To Life
Drug development usually begins with small steps. Teams might look to modify a promising molecule by attaching new pieces—hoping one switch unlocks a fresh therapy against cancer or infection. 4-Bromochlorobenzene opens those doors. Its presence helps scientists quickly build libraries of new molecules, each just a tweak away from the last. The more compounds a team can screen, the better chance they hit on something that changes lives for the better.
Agriculture uses the same principle. Picture designing a new crop protection agent—maybe a safer weed killer. Chemists use 4-Bromochlorobenzene to explore how plants or pests react to small changes. A new substituent in place of the bromine or chlorine might mean less crop damage or reduced residue in soil. That feels like progress.
Challenges and Solutions in Handling
Working in a lab, one quickly learns that safety isn’t just a poster on the wall. 4-Bromochlorobenzene—like many chemicals—calls for care. Skin contact or inhalation may cause problems, so protective gear isn’t optional. Factories and universities track how much gets stored and make sure spills or mismanagement don’t happen. Waste procedures, air quality checks, and staff training all help keep accidents rare.
I can remember colleagues insisting every bottle stays locked or logged in a database. Once, a lax approach to labeling led to an unnecessary evacuation—not because this compound is the most dangerous, but confusion just isn’t worth the risk. Tighter protocols and digital record-keeping now make slips less likely.
Looking Ahead: Better Chemistry With Fewer Hazards
The goal isn’t just more chemistry—it’s better chemistry. Green chemistry pushes researchers to swap out hazardous substances for safer ones or refine their methods to produce less waste. Projects that rely on 4-Bromochlorobenzene and similar compounds don’t escape this. Newer methods might someday let teams make the same transformations with enzymes or less harmful reagents.
A future where people can create medicines and materials without so much environmental burden or risk sits within reach, but it starts with honest conversations and steady improvements. Every bottle in a lab tells a story of how science advances, one compound at a time.
Chemistry in Simple Terms
Some chemicals cause headaches for people new to organic chemistry. 4-Bromochlorobenzene sounds intimidating, but the facts break down pretty clearly. The name says a lot about the structure. Take a regular benzene ring, the backbone of countless industrial, pharmaceutical, and research-grade molecules. Attach both a bromine atom and a chlorine atom, each to a separate carbon on the ring, at opposite sides. That "4-" in the name really just marks the para position: if you count from where one atom sits, the other lands right across from it. The molecular formula for this setup is simple to remember: C6H4BrCl.
Diving Deeper: How Atoms Link Up
Grasping the structure matters for anyone handling, synthesizing, or studying this compound. Picture a hexagon, each corner a carbon atom. On two opposite points, start with hydrogen, replace one with bromine, then swap another, directly across, with chlorine. The rest stick with hydrogen. Both halogens bond with the aromatic system. The shorthand form looks like this:
Br–C6H4–Cl (with Br and Cl in the 1 and 4 positions, respectively)
Experience in a basic university lab makes it clear why this matters. The spots where you put those halogens change not just the chemical’s reactivity but sometimes its toxicity and usefulness. The 1,4- (para) positioning, for instance, can help with separation during purification or when making more complex products. Molecules that look nearly identical, but place their atoms differently, often behave in real-world settings in totally new ways.
Why It Should Matter Outside Academia
Outside the classroom, the real draw centers on practical uses. Chemists rely on 4-Bromochlorobenzene to synthesize other compounds, especially in pharmaceuticals and in making advanced materials. Halogenated benzenes play roles as intermediates that let researchers build more sophisticated molecules, especially when switching halogens for new functional groups. In electronics, related compounds show up in liquid crystals or serve as starting ingredients for dye and pigment manufacturers.
There’s a risk side to this as well. Anyone working around halogenated aromatics faces questions of workplace safety and waste. There’s little room for error: both bromine- and chlorine-containing organics can resist natural breakdown, showing up in the environment long after disposal. Regulations on handling, storage, and disposal often reflect this, especially in advanced labs or manufacturing sites. Getting the formula and structure right saves time figuring out the right protocols.
Some Steps Toward Smarter Use
Controlling exposure starts with clear labeling and careful tracking from the time it arrives all the way to its use and disposal. Many labs provide extensive training on gloves, ventilation, and handling of spills for halogenated aromatics. Waste often heads out under hazardous waste permits.
On the research side, people look for greener reactions to create these sorts of molecules. New catalysts, milder conditions, and reduced waste all figure into responsible chemistry. Rather than ignore what happens at the end of a product’s life, smart labs think through not just the reaction yield but what will happen to every byproduct. Understanding the structure of 4-Bromochlorobenzene, even at a basic level, gives everyone a stronger foundation to make safer, more reliable decisions–from university students to industrial chemists.
Chemical Exposure: Hidden in Plain Sight
4-Bromochlorobenzene sounds like something you’d only find in a chemistry classroom, but it appears anywhere from research labs to chemical manufacturing sites. My time working alongside graduate students in a university lab taught me pretty quickly that chemicals with names like this deserve full respect for the harm they can cause. A clear, colorless liquid doesn’t seem threatening, but most accidents don’t look dangerous right away.
Health Hazards Aren’t Always Obvious
I remember reading material safety data sheets with a sense of routine, but the warnings stay with you. The truth about 4-Bromochlorobenzene is this: its vapor can irritate the nose, throat, and lungs. Reports from the National Institute for Occupational Safety and Health point to eye pain, dizziness, and nausea in poorly ventilated spaces. Once a chemical causes this kind of response, it’s worth treating every spill and every open bottle like it matters. Some studies link exposure to liver or kidney problems if contact becomes chronic.
The Realities of Environmental Risk
Letting this chemical slip down a drain means playing with consequences. 4-Bromochlorobenzene doesn’t break down quickly. It lingers in water, builds up in soil, and can move up aquatic food chains. During a summer internship at a waste management facility, I watched staff go to extra lengths to contain and recover halogenated organics like this. They understood that once chemicals like these hit the environment, nobody gets an easy fix. Its persistence makes it a bigger headache than more biodegradable compounds.
Evidence Behind Safe Handling
Looking at data from the European Chemicals Agency and EPA, strict limits exist for how workers handle this compound. Air levels in manufacturing plants get tested often to make sure people don’t inhale too much. Personal protective equipment—good gloves, goggles, industrial hoods—doesn’t come off just because nobody’s watching. Laboratories tend to lock bottles of 4-Bromochlorobenzene in fume hoods for a reason. These steps aren’t just about ticking safety boxes, they actually reduce accident rates and prevent trips to the ER.
Thinking About Safer Choices
I’ve seen some groups start looking for alternatives, especially if a chemical’s only advantage is price or ease of synthesis. Finding substitutes that are less toxic or break down more quickly often costs more, but long-term savings come in fewer accidents, less pollution, and smoother cleanups. At conferences, seasoned chemists share stories where a safer reagent made everyone’s life easier in the end—even if it seemed more expensive or complicated up front.
Shared Responsibility in Chemical Safety
Cutting corners isn’t just risky for lab coats and scientists. Anyone who transports, stores, or disposes of 4-Bromochlorobenzene owns a share of the problem and the solution. Better labeling, tougher spill procedures, and environmental monitoring bring peace of mind—never guaranteed, but always earned. Experience taught me that safety isn’t just about the rare disaster; it’s about stacking up small, everyday choices until nothing slips through the cracks. Taking 4-Bromochlorobenzene lightly is a shortcut nobody wants to pay for later.
Why 4-Bromochlorobenzene Calls for Caution
4-Bromochlorobenzene gives off a false sense of security because it looks like a harmless, clear solid. Folks in the lab or on the shop floor know that solids like this can still pack a punch, especially when dealing with halogenated aromatic compounds. Even without a dramatic warning sign, smart handling and smart storage go a long way to prevent long-term health headaches and property damage.
Proper Storage: Keeping Safety First
Anyone who's ever juggled a flammable or toxic substance knows the drill. For 4-Bromochlorobenzene, keep it cool, dry, and shielded from sunlight. Rooms with a steady temperature work best since wild swings make chemicals sweat and sometimes degrade. Humidity can lead to unwanted clumping or, worse, reactions with trace moisture. Chemical storerooms with proper ventilation and no exposure to open flames or extreme heat reduce the risk of accidental ignition or pressure buildup.
Always store this compound in tightly sealed glass or high-quality plastic containers—metal’s a no-go since halogenated organics and reactive metals can react over time. Stack containers on shelves that stop spills from spreading, and label every jar with both the name and hazard class so there’s never a guessing game.
Protective Equipment and Daily Handling
Chemical safety gear isn’t only for show. Start with nitrile or neoprene gloves, sturdy enough to stop skin contact for this compound. Eye protection keeps sneaky splashes out, and a lab coat shields your clothes and skin. I’ve learned that a set of clothes and gloves at the ready close to the handling site cuts down on accidents. Folks handling 4-Bromochlorobenzene should avoid eating or drinking nearby, since a stray hand can easily contaminate lunch or coffee.
The chemical doesn’t jump out of its bottle, but small spills or dust clouds aren’t uncommon. A fume hood or well-ventilated area keeps vapors away from your lungs. Inhalation over time can mess with your health, even when you don’t notice any immediate effect. Washing hands before leaving the work area might sound simple, but it prevents accidental spread to doorknobs, pens, or phones.
What to Do With Waste and Spills
Disposing of 4-Bromochlorobenzene takes more than a trash can or a sink. Arrange for certified hazardous waste disposal. Keep spills contained using absorbent pads or sand, and work from the outside in to stop the mess from getting bigger. Scoop up waste into a sealed container and label it straight away. Spills, even small ones, go into logs so no incident slips under the radar.
Potential Hazards and Emergency Response
On the rare occasion of a fire, standard water spray won’t always cut it. Dry chemical powder, CO2, or foam extinguishers work better. The chemical doesn’t tend to explode, but it releases hydrogen halides if burned, creating toxic clouds. Know the emergency exits and have a leak/chemical spill kit on standby. If you or a coworker gets some on the skin or in the eyes, rinse under running water for several minutes and seek medical attention.
Improving Workplace Chemical Safety
A quick review before every use—reading the safety data sheet and refreshing on procedures—acts like a backup plan. Investing in proper storage, keeping inventory records, and updating training for handling hazardous organics all contribute to a safer workplace. Continuous education, paired with a healthy sense of respect for these compounds, helps prevent costly or damaging mistakes.
Digging Deeper Into the Source
Some folks ask where they can buy or get hold of 4-Bromochlorobenzene, a chemical that shows up in labs and industry for making dyes, medicines and specialty materials. Behind this simple question stands a world of legal requirements, safety challenges, and genuine need for transparency.
Regulations Keep Us Honest
Sourcing any specialty chemical—let alone a halogenated benzene compound—means following the rules. You’ve got to know local and international law around chemical purchases and transport. In the US, suppliers won’t just hand over 4-Bromochlorobenzene because you click “add to cart.” Buyers prove their business legitimacy and sometimes explain the intended use. The European Union runs REACH, which screens for safety and legitimacy as well. These are not empty hurdles; accidents and poor oversight lead to environmental spills, criminal misuse, and real harm.
Trusted Suppliers and Why They Matter
From my own experience, buying solvents and fine chemicals never starts at eBay. Most reputable labs work with approved chemical distributors. Sigma-Aldrich, Alfa Aesar, TCI, and some smaller specialty firms set clear standards for purity, documentation, and traceability. If a supplier won’t show a Certificate of Analysis or hesitates over your business tax ID, steer clear. Real suppliers ask questions. They keep logs of sales. This protects their reputation, your safety, and the broader community.
The Real Cost: Beyond the Price Tag
Many forget that sourcing comes with additional chores—storage, compliance, and waste management. 4-Bromochlorobenzene isn’t explosive, but it’s not a snack food either. We keep it away from drains, children, and amateur hands because education and preparation matter more than low prices. I’ve seen newcomers trip over disposal requirements and face fines that dwarf any savings from sketchy sources.
Why Labs and Makers Need These Chemicals
Specialty chemicals like this drive new materials, pharmaceuticals and research on the next generation of coatings or polymers. If you work in a university or a licensed business, you’ll usually have access to purchasing channels. Lost access stunts progress and limits what researchers can do. But no one should cut corners for speed or price. In an honest lab, shortcuts usually end in legal trouble or at least garbage data.
Looking for Solutions
Solving the puzzle of legal, reliable access to specialty chemicals could work better with centralized vetting and easier transparency. Streamlined digital safety training, better public guidance, and industry-wide record keeping would help. Instead of a tangled web of obscure gatekeeping, an online registry for accredited buyers might cut red tape while keeping communities safe.
Takeaway from the Lab
Every time I see a question about buying 4-Bromochlorobenzene, I remember those first nerve-wracking days of filling out order forms and checking storage cabinets twice. Find local laws, pick a reputable supplier, keep records straight, and always respect the chemical itself. The science world grows stronger if everyone follows the same honest, cautious path.